

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:50
What does standard deviation reveal about data? a. the average of all the data points b. which of the data points is most reliable c. how spread out the data points are d. the percent error included in the data
Answers: 2


Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3
You know the right answer?
2 C6H14 + 19 O2 --> 12 CO2 + 14 H2O
Given 250 g of Oxygen and 50 grams of CoH14 what is the maxi...
Questions


English, 07.12.2020 21:00

History, 07.12.2020 21:00

Physics, 07.12.2020 21:00

Mathematics, 07.12.2020 21:00




Mathematics, 07.12.2020 21:00


Mathematics, 07.12.2020 21:00

Mathematics, 07.12.2020 21:00



Business, 07.12.2020 21:00



Mathematics, 07.12.2020 21:00

Biology, 07.12.2020 21:00

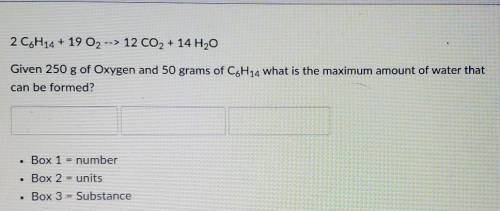
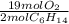 = 5.51 mol O₂
= 5.51 mol O₂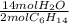 = 4.06 mol H₂O
= 4.06 mol H₂O


