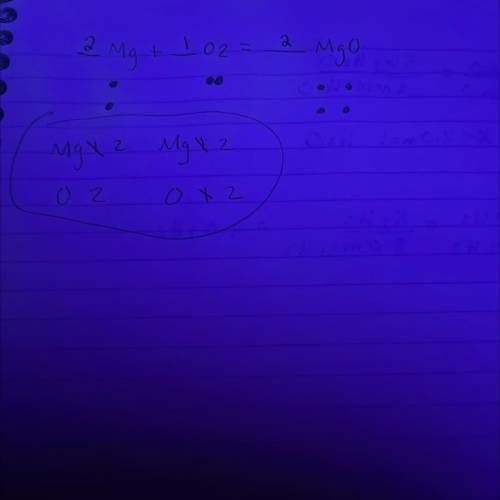In a balanced chemical reaction, the number of atoms of each element in the product(s) always equals the
A. molar mass of the reactants
B. proportional masses of the reactants
C. the number of atoms of each element in the reatants
D. the number of elements in the reactants

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1

Chemistry, 22.06.2019 15:00
‘which reaction would most likely require the use of an inert electrode?
Answers: 1

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2
You know the right answer?
In a balanced chemical reaction, the number of atoms of each element in the product(s) always equals...
Questions



Biology, 10.12.2020 22:20



Computers and Technology, 10.12.2020 22:20


Chemistry, 10.12.2020 22:20

Advanced Placement (AP), 10.12.2020 22:20

Mathematics, 10.12.2020 22:20


Mathematics, 10.12.2020 22:20


Mathematics, 10.12.2020 22:20

Chemistry, 10.12.2020 22:20

Mathematics, 10.12.2020 22:20

Mathematics, 10.12.2020 22:20


Mathematics, 10.12.2020 22:20