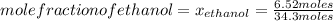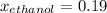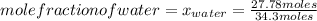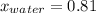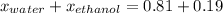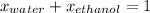Chemistry, 14.05.2021 03:10 joseperez1224
What is the mole fraction of each component in a solution made by mixing 300 g of ethanol(C2H5OH) and 500 g of water?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have metallic bonds. which of the following is most likely a property of this substance? a. low conductivity b. low boiling point c. high malleability d. high solubility in water
Answers: 2
You know the right answer?
What is the mole fraction of each component in a solution made by mixing 300 g of ethanol(C2H5OH) an...
Questions


English, 25.02.2021 18:40



Mathematics, 25.02.2021 18:40


Chemistry, 25.02.2021 18:40




Mathematics, 25.02.2021 18:40


History, 25.02.2021 18:40

History, 25.02.2021 18:40

Chemistry, 25.02.2021 18:40



Mathematics, 25.02.2021 18:40


World Languages, 25.02.2021 18:40

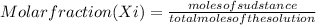
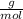 Water 18
Water 18 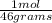 = 6.52 moles
Moles of water: 500 grams*
= 6.52 moles
Moles of water: 500 grams* 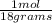 = 27.78 moles
= 27.78 moles