
Chemistry, 13.05.2021 22:20 angieplasencia8
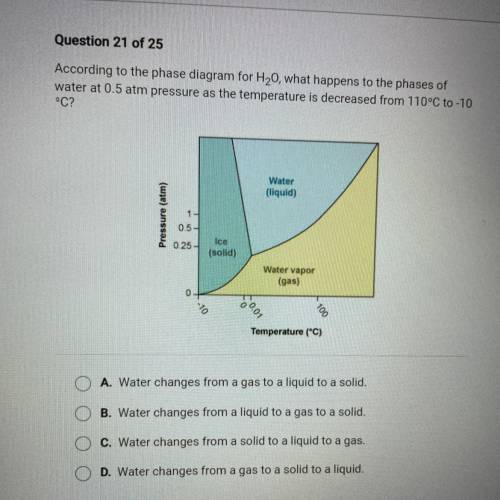
According to the phase diagram for H20, what happens to the phases of
water at 0.5 atm pressure as the temperature is decreased from 110°C to -10
°C?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2
You know the right answer?
According to the phase diagram for H20, what happens to the phases of
water at 0.5 atm pressure as...
Questions


Mathematics, 12.10.2019 01:30

Business, 12.10.2019 01:30


Physics, 12.10.2019 01:30


Mathematics, 12.10.2019 01:30

Arts, 12.10.2019 01:30



Chemistry, 12.10.2019 01:30

Social Studies, 12.10.2019 01:30


Mathematics, 12.10.2019 01:30


Social Studies, 12.10.2019 01:30

Mathematics, 12.10.2019 01:30

Computers and Technology, 12.10.2019 01:30

Biology, 12.10.2019 01:30

Mathematics, 12.10.2019 01:30



