
Chemistry, 13.05.2021 22:00 mettababeeeee
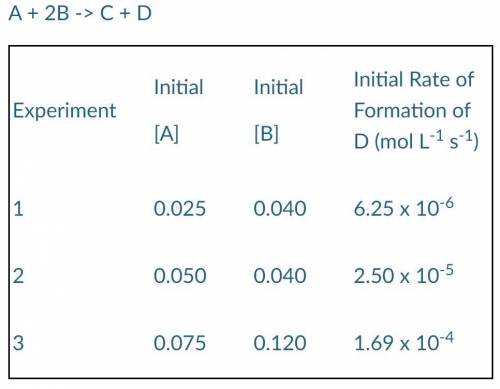
The following results were obtained when the reaction represented above was studied at 25°C:
A + 2B -> C + D
_
a) Determine the order of the reaction with respect to A and B.
b) Write the rate law for the reaction.
c) Calculate the value of the rate constant, k, specifying units.
d) Determine the initial rate of disappearance of substance B in Experiment 2.
_
e) Identify which of the reaction mechanisms represented below is consistent with the rate law developed in part B. Justify your choice by writing rate laws for each mechanism. Also, identify any intermediate(s) and/or catalyst(s) in your chosen mechanism. (6 pts - 1 pt for chosen mechanism, 1 pt for each rate law, 2 pts for intermediate(s)/catalyst(s))
_
Mechanism #1: A -> M (slow)
M + B -> C + X (fast)
X + B -> D (fast)
Mechanism #2: B ⇋ M (fast equilibrium)
M + A -> C + X (slow)
B + X -> D (fast)
Mechanism #3: A + B ⇋ M (fast equilibrium)
M + B -> C + X (slow)
X -> D (fast)
_

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2


Chemistry, 23.06.2019 01:00
What is the chemical name of the compound ti2o3? use the list of polyatomic ions and the periodic table to you answer.
Answers: 1

Chemistry, 23.06.2019 06:00
Which factor is likely to impact the possible number of compounds? presence of unlimited number of elements in the periodic table the inability of atoms to align perfectly with other atoms the ability of all elements to react with every other element all elements being equally reactive
Answers: 2
You know the right answer?
The following results were obtained when the reaction represented above was studied at 25°C:
A + 2B...
Questions


Mathematics, 09.10.2021 14:00

Mathematics, 09.10.2021 14:00


Mathematics, 09.10.2021 14:00




Mathematics, 09.10.2021 14:00

Mathematics, 09.10.2021 14:00

Chemistry, 09.10.2021 14:00

Mathematics, 09.10.2021 14:00

English, 09.10.2021 14:00




Mathematics, 09.10.2021 14:00

Mathematics, 09.10.2021 14:00





