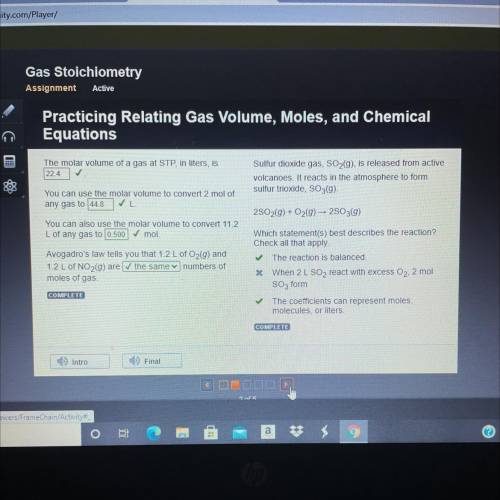
The molar volume of a gas at STP, in liters, is
22.4 ✓
You can use the molar volume to conver...

The molar volume of a gas at STP, in liters, is
22.4 ✓
You can use the molar volume to convert 2 mol of
any gas to 44.8
You can also use the molar volume to convert 11.2
L of any gas to 0.500
mol.
Avogadro's law tells you that 1.2 L of O2(9) and
1.2 L of NO2(9) are the same numbers of
moles of gas.
COMPLETE

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Water is important for the of cells. a: size, shape, and temperature b: temperature, color, and odor c: color, odor, and size d: shape, temperature, and color
Answers: 2


Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1
You know the right answer?
Questions

Health, 18.07.2020 17:01















Computers and Technology, 18.07.2020 17:01



Computers and Technology, 18.07.2020 17:01



