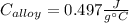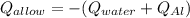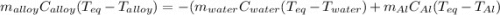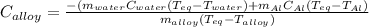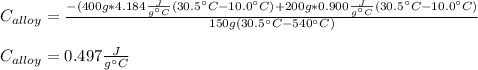Chemistry, 13.05.2021 20:40 ptanner706
A 0.150-kg sample of a metal alloy is heated at 540 Celsius an then plunged into a 0.400-kg of water at 10.0 Celsius, which is contained in a 0.200-kg aluminum calorimeter cup. The final temperature of the system is 30.5 Celsius. What is the specific heat of the metal alloy in J/Kg. Celsius

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 03:10
The peak wavelength for the blackbody curve of a star is in the uv range. assuming the radiation from this star can reach earth, would you be able to see it?
Answers: 2

Chemistry, 22.06.2019 10:30
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3

You know the right answer?
A 0.150-kg sample of a metal alloy is heated at 540 Celsius an then plunged into a 0.400-kg of water...
Questions

Social Studies, 23.09.2020 15:01

Biology, 23.09.2020 15:01







English, 23.09.2020 15:01



Mathematics, 23.09.2020 15:01

Chemistry, 23.09.2020 15:01

Mathematics, 23.09.2020 15:01

Mathematics, 23.09.2020 15:01





Chemistry, 23.09.2020 15:01