
Chemistry, 13.05.2021 06:20 supermansabeast
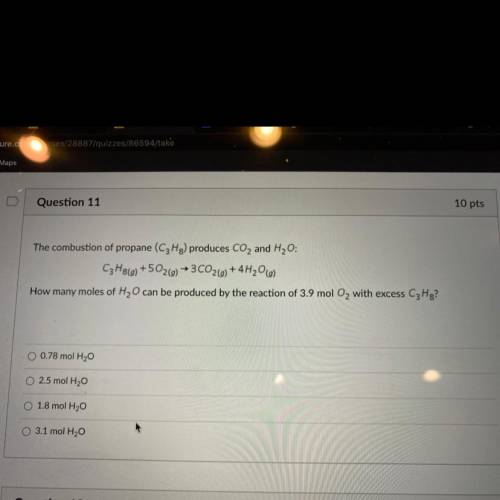
The combustion of propane (C3Hg) produces CO2 and H2O:
C3H8() +502(g) → 3CO2(g) + 4H20(g)
How many moles of H2O can be produced by the reaction of 3.9 mol O2 with excess C3H8?

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3


Chemistry, 23.06.2019 06:00
•what conclusions can you make about the relationship between the volume of a gas and its temperature? • what conclusions can you make about the relationship between the volume of a gas and its pressure? • what possible variables have you not accounted for? as you did the procedures, is it possible that the atmospheric pressure may have changed? if it did change over the course of your experiment, then how would your results have been affected?
Answers: 3
You know the right answer?
The combustion of propane (C3Hg) produces CO2 and H2O:
C3H8() +502(g) → 3CO2(g) + 4H20(g)
How...
How...
Questions


English, 10.10.2019 00:20




Mathematics, 10.10.2019 00:20


Geography, 10.10.2019 00:20














