
Chemistry, 13.05.2021 03:40 jesussaves333
Which of the following reactions is exothermic? A. light + 6CO2 + 6H2O → C8H12O6 + 6O2 B. 2HgO (s) + heat → 2Hg(I) + O2(g) C. Mercury oxide plus heat yields mercury and oxygen D. None of the reactions are exothermic.
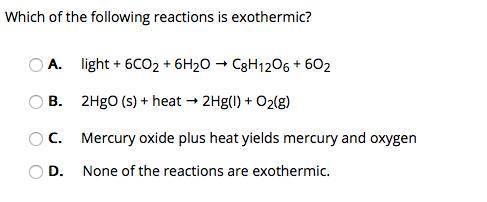

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2

Chemistry, 23.06.2019 01:30
What is the importance of interlocking the fingers and rubbing while washing hands? the palms are the dirtiest parts of the hands. the spaces between the fingers get washed. the backs of the hands get washed. the fingernails are the dirtiest parts of the hands
Answers: 1

Chemistry, 23.06.2019 03:30
The semi-conductors on the periodic table are classified as
Answers: 1
You know the right answer?
Which of the following reactions is exothermic? A. light + 6CO2 + 6H2O → C8H12O6 + 6O2 B. 2HgO (s) +...
Questions

Advanced Placement (AP), 21.09.2019 19:00

Mathematics, 21.09.2019 19:00

Social Studies, 21.09.2019 19:00









Mathematics, 21.09.2019 19:00


Mathematics, 21.09.2019 19:00

Mathematics, 21.09.2019 19:00

Advanced Placement (AP), 21.09.2019 19:00



History, 21.09.2019 19:00

Mathematics, 21.09.2019 19:00



