
Chemistry, 13.05.2021 01:10 coolestkid2401
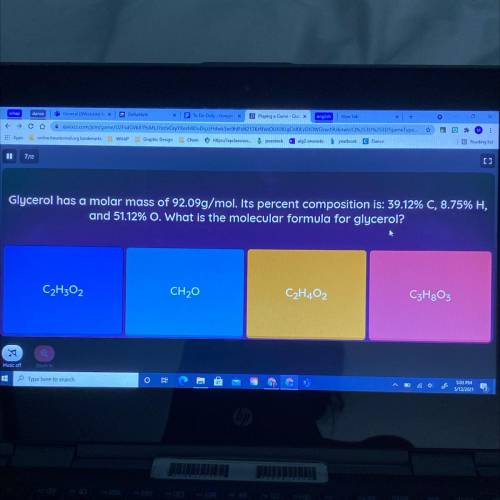
Glycerol has a molar mass of 92.09g/mol. Its percent composition is: 39.12% C, 8.75% H, and 51.12% O. What is the molecular formula for glycerol?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 23.06.2019 00:30
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1
You know the right answer?
Glycerol has a molar mass of 92.09g/mol. Its percent composition is: 39.12% C, 8.75% H,
and 51.12%...
Questions



Mathematics, 03.05.2021 21:40

Mathematics, 03.05.2021 21:40


Mathematics, 03.05.2021 21:40


Mathematics, 03.05.2021 21:40


History, 03.05.2021 21:40

Social Studies, 03.05.2021 21:40

Mathematics, 03.05.2021 21:40


Mathematics, 03.05.2021 21:40

Mathematics, 03.05.2021 21:40

Mathematics, 03.05.2021 21:40

English, 03.05.2021 21:40



Health, 03.05.2021 21:40



