
Chemistry, 13.05.2021 01:00 jasonr182017
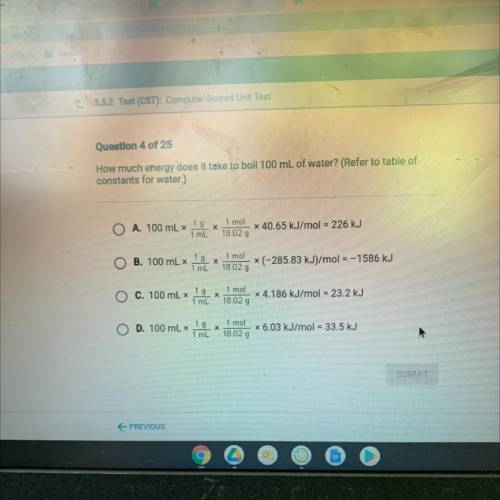
How much energy does it take to boil 100 mL of water? (Refer to table of
constants for water.)
A. 100 mL x
1 g
х
1 mL
1 mol
18.02 g
x 40.65 kJ/mol = 226 kJ
B. 100 mL x
1 g
1 mL
X
1 mol
18.02 g
(-285.83 kJ)/mol = –1586 kJ
C. 100 mL x
1 g
X
1 mol
18.02 g
x 4.186 kJ/mol = 23.2 kJ
1 mL
1 g
D. 100 mL x
1 mL
1 mol
x
18 02 g
x 6.03 kJ/mol = 33.5 kJ

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 1

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2
You know the right answer?
How much energy does it take to boil 100 mL of water? (Refer to table of
constants for water.)
Questions




History, 08.04.2022 06:10

Mathematics, 08.04.2022 06:20



Mathematics, 08.04.2022 06:50



Mathematics, 08.04.2022 06:50


Mathematics, 08.04.2022 07:00



Physics, 08.04.2022 07:30


SAT, 08.04.2022 07:50

SAT, 08.04.2022 07:50




