
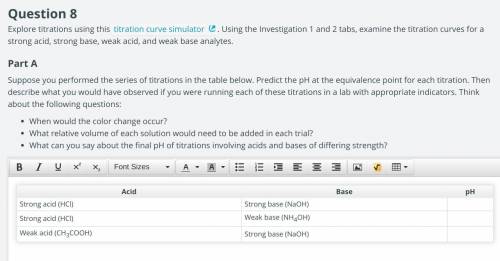
Suppose you performed the series of titrations in the table below. Predict the pH at the equivalence point for each titration. Then describe what you would have observed if you were running each of these titrations in a lab with appropriate indicators. ill give the link if needed

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 23.06.2019 00:00
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3

Chemistry, 23.06.2019 04:00
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2
You know the right answer?
Suppose you performed the series of titrations in the table below. Predict the pH at the equivalence...
Questions


Chemistry, 25.11.2020 16:30

Mathematics, 25.11.2020 16:30

Mathematics, 25.11.2020 16:30

Chemistry, 25.11.2020 16:30

Mathematics, 25.11.2020 16:30


History, 25.11.2020 16:30

Physics, 25.11.2020 16:30


Mathematics, 25.11.2020 16:30


Business, 25.11.2020 16:40




Health, 25.11.2020 16:40


Chemistry, 25.11.2020 16:40



