
Chemistry, 12.05.2021 17:40 mariahbugg7
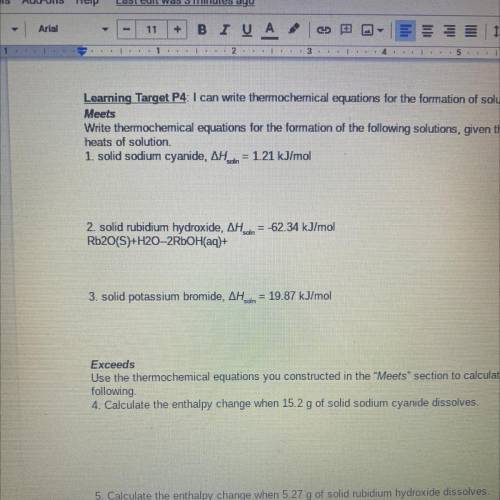
Write thermochemical equations for the formation of the following solutions, given their molar
heats of solution.
1. solid sodium cyanide, AH. din = 1.21 kJ/mol
2. solid rubidium hydroxide, AH dn = -62.34 kJ/mol
Rb2O(S)+H20-2RbOH(aq)+
I
3. solid potassium bromide, AH..in = 19.87 kJ/mol
Exceeds
Use the thermochemical equations you constructed in the "Meets" section to calculate the

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3
You know the right answer?
Write thermochemical equations for the formation of the following solutions, given their molar
heat...
Questions

Mathematics, 05.03.2021 01:00


Mathematics, 05.03.2021 01:00


Mathematics, 05.03.2021 01:00

Mathematics, 05.03.2021 01:00

French, 05.03.2021 01:00

Geography, 05.03.2021 01:00

Health, 05.03.2021 01:00

Mathematics, 05.03.2021 01:00

Mathematics, 05.03.2021 01:00

Biology, 05.03.2021 01:00

Mathematics, 05.03.2021 01:00


Biology, 05.03.2021 01:00

History, 05.03.2021 01:00



Geography, 05.03.2021 01:00




