
Chemistry, 12.05.2021 14:00 joelpimentel
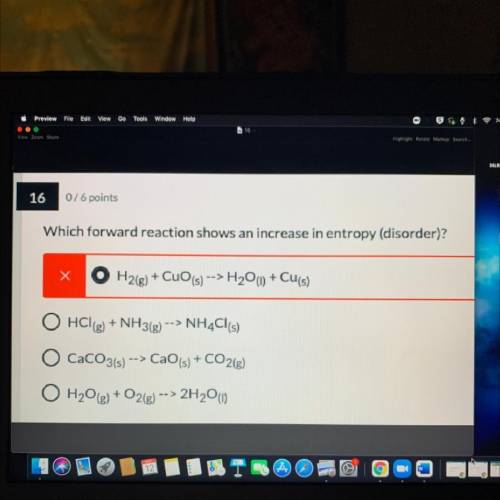
Which forward reaction shows an increase in entropy (disorder)?explain
A. H2(g) + CuO (s) --> H2O(l) + Cu(s)
B. HCl(g) + NH3(g) --> NH4Cl(s)
C. CaCO3(s) -> CaO(s) + CO2g)
D. H2O(g) + O2(g) --> 2H2O)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 00:30
Maria wants to determine which type of disinfectant kills the most bacteria. which of the following is the best way for maria to determine this? a. ask ten different companies that make disinfectants which type is best. b. put the same amount and species of bacteria on ten identical plates, and add ten different kinds of disinfectant to each plate. c. interview ten different people to determine which type of disinfectant they prefer. d. put the same amount and species of bacteria on ten identical plates, and add a different disinfectant to each plate.
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1
You know the right answer?
Which forward reaction shows an increase in entropy (disorder)?explain
A. H2(g) + CuO (s) --> H2...
Questions

Mathematics, 25.03.2021 23:30

English, 25.03.2021 23:30


Computers and Technology, 25.03.2021 23:30


Mathematics, 25.03.2021 23:30

Biology, 25.03.2021 23:30

Chemistry, 25.03.2021 23:30

Physics, 25.03.2021 23:30

Mathematics, 25.03.2021 23:30


Biology, 25.03.2021 23:30

English, 25.03.2021 23:30


Mathematics, 25.03.2021 23:30

Mathematics, 25.03.2021 23:30

Spanish, 25.03.2021 23:30

Mathematics, 25.03.2021 23:30

Mathematics, 25.03.2021 23:30




