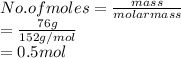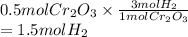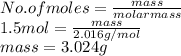Chemistry, 12.05.2021 01:50 PONBallfordM89
A chemist uses hot hydrogen gas to convert chromium(III) oxide to
pure chromium. How many grams of hydrogen are needed to
convert 76 grams of chromium(III) oxide, Cr203?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3


Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

You know the right answer?
A chemist uses hot hydrogen gas to convert chromium(III) oxide to
pure chromium. How many grams of...
Questions

History, 22.12.2021 14:30

Mathematics, 22.12.2021 14:30



Mathematics, 22.12.2021 14:30


Physics, 22.12.2021 14:30




Mathematics, 22.12.2021 14:30





English, 22.12.2021 14:30




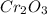
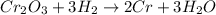
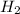 .
. 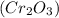 is 152 g/mol.
is 152 g/mol.