X-29: 28.976 amu; 4.68%

Chemistry, 11.05.2021 23:10 andrewbigbrains8740
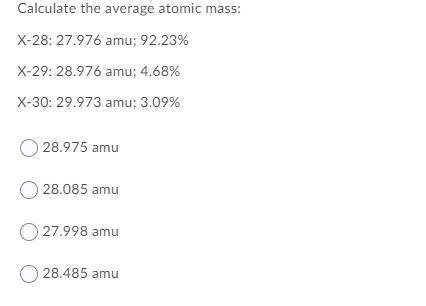
Calculate the average atomic mass:
X-28: 27.976 amu; 92.23%
X-29: 28.976 amu; 4.68%
X-30: 29.973 amu; 3.09%

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1

Chemistry, 23.06.2019 01:00
Which statement best describes isomers? a. isomers are alcohols that have the same functional group. b. isomers have at least one carbon-carbon double bond. c. isomers have the same molecular formula but different structural properties.
Answers: 1

Chemistry, 23.06.2019 05:40
Convert a speed of 201 cm/s to units of inches per minute. also, show the unit analysis by dragging components into the unit‑factor slots.
Answers: 1
You know the right answer?
Calculate the average atomic mass:
X-28: 27.976 amu; 92.23%
X-29: 28.976 amu; 4.68%
X-29: 28.976 amu; 4.68%
Questions



Mathematics, 24.11.2020 06:40

Advanced Placement (AP), 24.11.2020 06:40

Mathematics, 24.11.2020 06:40


History, 24.11.2020 06:40


Computers and Technology, 24.11.2020 06:40

Mathematics, 24.11.2020 06:40



Computers and Technology, 24.11.2020 06:40


Business, 24.11.2020 06:50

Computers and Technology, 24.11.2020 06:50

Chemistry, 24.11.2020 06:50



Chemistry, 24.11.2020 06:50



