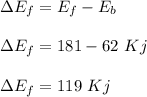Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:50
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1

Chemistry, 22.06.2019 04:30
How do i complete this electrolysis of water lab? i’m at home, so i don’t have the materials, and the lab didn’t properly work and was incomplete at school.
Answers: 1

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1
You know the right answer?
For a chemical reaction, the activation energy for the forward reaction is +181 kJ and the activatio...
Questions





Social Studies, 13.12.2021 20:20


Mathematics, 13.12.2021 20:20

Physics, 13.12.2021 20:20


Chemistry, 13.12.2021 20:20

World Languages, 13.12.2021 20:20

Physics, 13.12.2021 20:20





Mathematics, 13.12.2021 20:20