
Chemistry, 11.05.2021 18:20 princesskhj6932
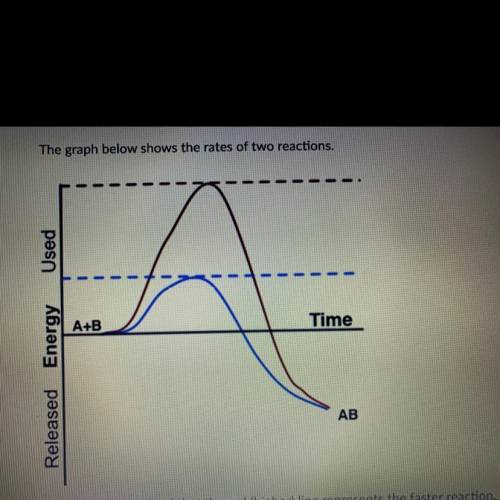
Student A determined that the red (higher) line represents the faster reaction.
Student B determined that the blue (lower) line represents the faster reaction.
Which student is correct and why? Be sure to specifically use information from the
graph to support your answer.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Explain which group an element with the electron configuration 1s2 2s2 2p6 3s2 3p6 3d1 4s2 belongs to.
Answers: 3

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2
You know the right answer?
Student A determined that the red (higher) line represents the faster reaction.
Student B determine...
Questions

Mathematics, 18.09.2021 03:10











English, 18.09.2021 03:10


Engineering, 18.09.2021 03:10


History, 18.09.2021 03:10






