Answers: 1


Another question on Chemistry


Chemistry, 23.06.2019 07:50
What is the significance sodium hydroxide and hydrochloric acid
Answers: 1

Chemistry, 23.06.2019 09:00
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate according to the following equation:
Answers: 2

Chemistry, 23.06.2019 12:30
Question 1 (true/false worth 4 points) (03.06 lc) an induced dipole occurs when a molecule's moving electrons are briefly more concentrated in one place than another, causing the molecule to become temporarily polarized. true false
Answers: 2
You know the right answer?
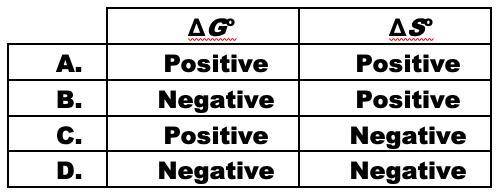
When ammonium nitrate dissolves in water, the solution becomes cold.
NH4NO3(s) <-> NH4+(aq)...
Questions



English, 12.03.2020 08:08

Chemistry, 12.03.2020 08:09

Mathematics, 12.03.2020 08:09


Health, 12.03.2020 08:09







Mathematics, 12.03.2020 08:11





History, 12.03.2020 08:12