
PLEASE NO BOTS lol
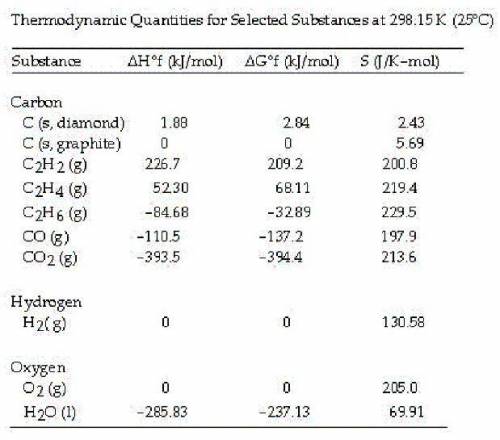
Ethyne or acetylene is also used in cutting torches. The acetylene is combined with pure oxygen producing a flame with a temperature of 6332 °F or 3500 °C. The combustion of acetylene in the presence of excess oxygen yields carbon dioxide and water:
2C2H2 (g) + 5O2 (g) --> 4CO2 (g) + 2H2O (l)
Calculate the value of ΔS° for this reaction.
A. +689.3 J/mol K
B. +432.4 J/mol K
C. -432.4 J/mol K
D. -122.3 J/mol K

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3


Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3
You know the right answer?
PLEASE NO BOTS lol
Ethyne or acetylene is also used in cutting torches. The acetylene is combined...
Questions



Social Studies, 12.07.2019 05:20

Mathematics, 12.07.2019 05:20

English, 12.07.2019 05:20


Mathematics, 12.07.2019 05:20


Mathematics, 12.07.2019 05:20













