
Chemistry, 10.05.2021 14:00 amiechap12
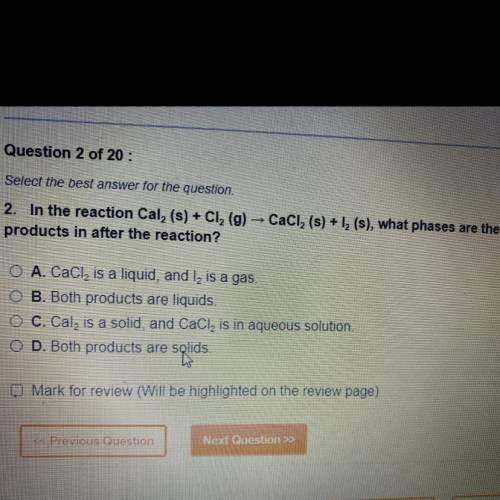
In the reaction Cal 2 (s)+Cl 2 (g) CaCl 2 (s)+l 2 (s) , what phases are the products in after the reaction ?
A. CaCl 2 is a liquid, and l2 is a gas
B. Both products are a liquid
C. Cal2 is a solid, CaCl2 is in aqueous solution
D. Both products are solids

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 10:00
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3


Chemistry, 22.06.2019 22:30
Astudent pours 10.0 g of salt into a container of water and observes the amount of time it takes for the salt to dissolve. she then repeats the process using the same amounts of salt and water but this time she slowly stirs the mixture while it is dissolving. the student performs the experiment one more time but this time she stirs the mixture rapidly. the dependent variable in this experiment is: time for salt to dissolve speed of stirring amount of water mass of salt
Answers: 1
You know the right answer?
In the reaction Cal 2 (s)+Cl 2 (g) CaCl 2 (s)+l 2 (s) , what phases are the products in after the re...
Questions

History, 12.10.2020 22:01




Mathematics, 12.10.2020 22:01

Mathematics, 12.10.2020 22:01

History, 12.10.2020 22:01



Chemistry, 12.10.2020 22:01

Mathematics, 12.10.2020 22:01



English, 12.10.2020 22:01





Geography, 12.10.2020 22:01




