
Chemistry, 08.05.2021 01:00 mckenziealexander
When sodium is placed in water, the following chemical reaction occurs. 2Na(s) + 2H2O (l) —> 2NaOH (aq) + H2 (g). If a teacher uses a 0.500 gram sample of sodium metal for a demonstration with excess water, how many liters of hydrogen gas would be produced at STP?
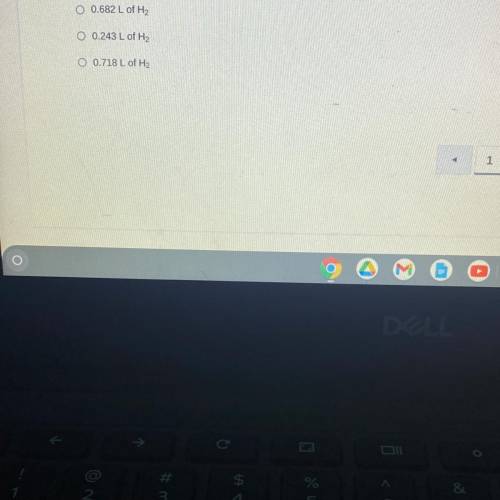

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The speed of light is around 6.706×10^8 miles per hour. what is the speed of light in units of miles per minute?
Answers: 2

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1


Chemistry, 23.06.2019 07:20
F1.5 mol of nabh4 react, how many moles of b2h6 are formed? 2 nabh4(aq) + h2so4(aq) → 2 h2(g) + na2so4(aq) + b2h6(g)
Answers: 1
You know the right answer?
When sodium is placed in water, the following chemical reaction occurs. 2Na(s) + 2H2O (l) —> 2NaO...
Questions




Biology, 04.03.2021 18:50










Mathematics, 04.03.2021 18:50

Mathematics, 04.03.2021 18:50



Biology, 04.03.2021 18:50

History, 04.03.2021 18:50



