
Chemistry, 08.05.2021 01:00 kennakenken3
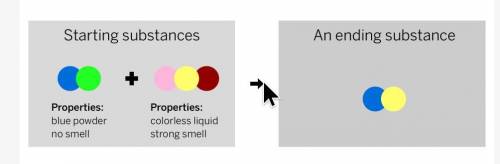
A chemist mixed two substances: a blue powder with no smell and a colorless liquid with a strong smell. Their repeating groups of atoms are shown above on the left. After they were mixed, the chemist analyzed the results and found two substances. One ending substance had the repeating group of atoms shown above on the right. Is the ending substance the same as the blue powder? What happened to the atoms of the starting substances when the ending substances formed? Be sure to explain your answers to both of these questions.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which best describes how johannes kepler developed his laws of planetary motion
Answers: 3

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3

Chemistry, 22.06.2019 20:30
A40 kilogram skier starts at the top of a 12 meter high slope. at the bottom, she is travelling 10 meters per second. how much energy does she lose to friction
Answers: 2

You know the right answer?
A chemist mixed two substances: a blue powder with no smell and a colorless liquid with a strong sme...
Questions


Mathematics, 19.08.2021 23:10

Spanish, 19.08.2021 23:10

History, 19.08.2021 23:10

Mathematics, 19.08.2021 23:10





Mathematics, 19.08.2021 23:10

Physics, 19.08.2021 23:10


Mathematics, 19.08.2021 23:10


Biology, 19.08.2021 23:10

Mathematics, 19.08.2021 23:10

Mathematics, 19.08.2021 23:10

History, 19.08.2021 23:10


Mathematics, 19.08.2021 23:10



