
Chemistry, 07.05.2021 22:10 BreadOfTheBear
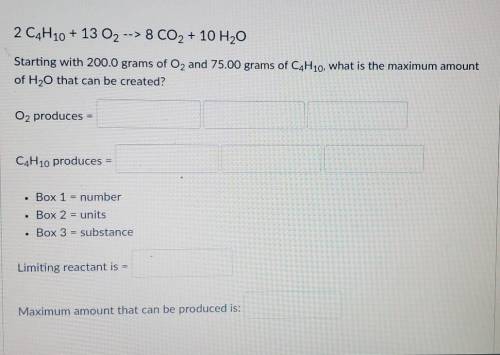
Starting with 200.0 grams of O2 and 75.00 grams of C4H10, what is the maximum amount of H2O that can be created?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3

Chemistry, 22.06.2019 23:30
The density of benzene at 15 °c is 0.8787 g/ml. calculate the mass of 0.1500 l of benzene at this temperature. enter your answer in terms of grams
Answers: 2

Chemistry, 23.06.2019 01:30
List and describe the neurological effects of the vocs and other air pollutants,as described by dr.theo colborn
Answers: 2
You know the right answer?
Starting with 200.0 grams of O2 and 75.00 grams of C4H10, what is the maximum amount of H2O that can...
Questions

Biology, 16.12.2020 21:50




Mathematics, 16.12.2020 21:50

Biology, 16.12.2020 21:50

Mathematics, 16.12.2020 21:50

Mathematics, 16.12.2020 21:50



Mathematics, 16.12.2020 21:50





Mathematics, 16.12.2020 21:50

Mathematics, 16.12.2020 21:50


English, 16.12.2020 21:50




