Someone please help!!
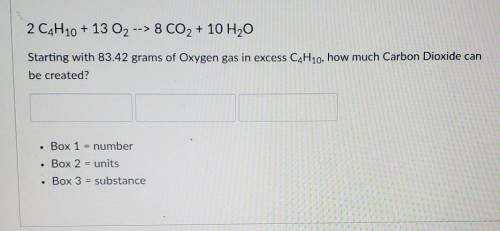
2 C4H10 + 13 O2 --> 8 CO2 + 10 H2O
Starting with 83.42 grams of Oxyg...

Chemistry, 07.05.2021 22:00 xMABRYx1991
Someone please help!!
2 C4H10 + 13 O2 --> 8 CO2 + 10 H2O
Starting with 83.42 grams of Oxygen gas in excess C4H10, how much Carbon Dioxide can be created?
Box 1 = number
Box 2 = units
Box 3 = substance

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:00
When a comet collides with earth, it adds material to our planet and causes great damage. therefore, a collision like this is a a. destructive force b. constructive force c. geologic process and event d. constructive and destructive force
Answers: 1



Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1
You know the right answer?
Questions


History, 04.02.2020 16:45


Arts, 04.02.2020 16:45


Mathematics, 04.02.2020 16:45

Mathematics, 04.02.2020 16:45

History, 04.02.2020 16:45

Mathematics, 04.02.2020 16:45



Mathematics, 04.02.2020 16:46

Mathematics, 04.02.2020 16:46









