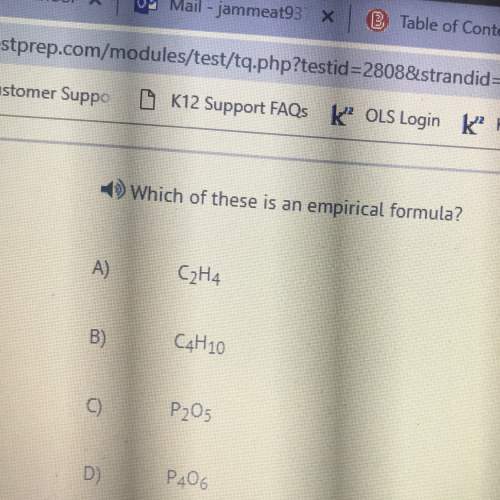
Chemistry, 07.05.2021 03:20 Honeyswish7730
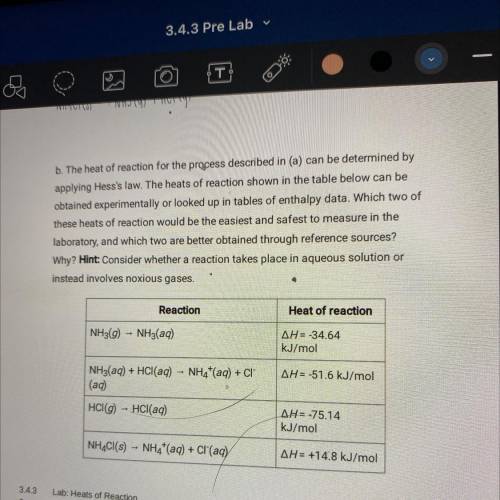
B. The heat of reaction for the process described in (a) can be determined by
applying Hess's law. The heats of reaction shown in the table below can be
obtained experimentally or looked up in tables of enthalpy data. Which two of
these heats of reaction would be the easiest and safest to measure in the
laboratory, and which two are better obtained through reference sources?
Why? Hint: Consider whether a reaction takes place in aqueous solution or
instead involves noxious gases.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 12:40
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2
You know the right answer?
B. The heat of reaction for the process described in (a) can be determined by
applying Hess's law....
Questions

English, 29.06.2020 15:01

Mathematics, 29.06.2020 15:01

Mathematics, 29.06.2020 15:01



Mathematics, 29.06.2020 15:01




Mathematics, 29.06.2020 15:01

English, 29.06.2020 15:01

Law, 29.06.2020 15:01

Physics, 29.06.2020 15:01


Mathematics, 29.06.2020 15:01

Biology, 29.06.2020 15:01

English, 29.06.2020 15:01


Computers and Technology, 29.06.2020 15:01