
Chemistry, 06.05.2021 05:40 aaliyahrice02
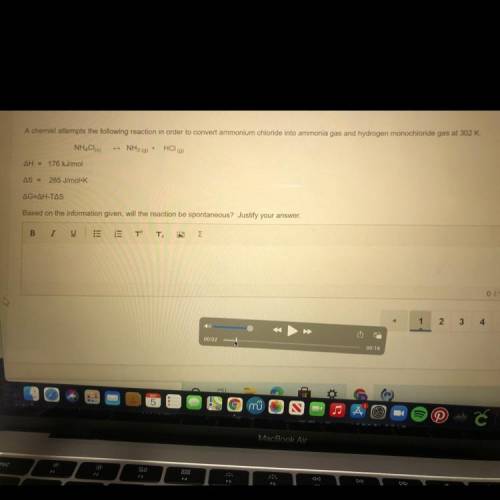
A chemist attempts the following reaction in order to convert ammonium chloride into ammonia gas and hydrogen monochloride gas at 302 K. Based on the information given, will the reaction be spontaneous? Justify your answer.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3



Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1
You know the right answer?
A chemist attempts the following reaction in order to convert ammonium chloride into ammonia gas and...
Questions


Mathematics, 19.09.2021 09:30


Mathematics, 19.09.2021 09:30


Mathematics, 19.09.2021 09:30




Mathematics, 19.09.2021 09:30


Arts, 19.09.2021 09:30

History, 19.09.2021 09:40

Biology, 19.09.2021 09:40

Geography, 19.09.2021 09:40




Mathematics, 19.09.2021 09:40



