
Chemistry, 06.05.2021 01:20 11needhelp11
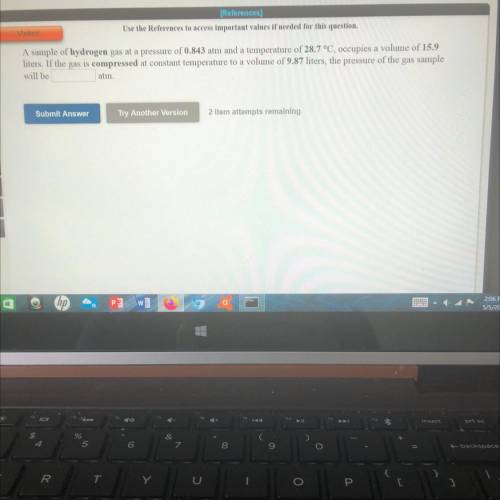
A sample of hydrogen gas at a pressure of 0.843 atm and a temperature of 28.7 °C, occupies a volume of 15.9
liters. If the gas is compressed at constant temperature to a volume of 9.87 liters, the pressure of the gas sample
will be
atm

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1


Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2

You know the right answer?
A sample of hydrogen gas at a pressure of 0.843 atm and a temperature of 28.7 °C, occupies a volume...
Questions

Mathematics, 06.03.2021 05:40





English, 06.03.2021 05:50

History, 06.03.2021 05:50

English, 06.03.2021 05:50


Mathematics, 06.03.2021 05:50

Chemistry, 06.03.2021 05:50


English, 06.03.2021 05:50

Business, 06.03.2021 05:50



History, 06.03.2021 05:50





