
Chemistry, 05.05.2021 21:40 jasperzhouzihe3018
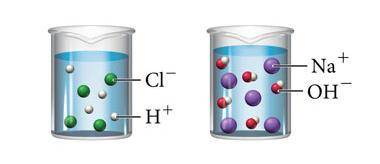
The image above shows what happens when hydrochloric acid (HCl) and sodium hydroxide (NaOH) are mixed with water. Which statement below is true about both solutions?
A. Both solutions contain solutes that do not dissolve completely in water.
B. Both solutions are saturated because they contain the maximum concentration of a solute dissolved in the solvent.
C. Both solutions will conduct an electric current because they both contain ions that can carry a charge.
D. Both solutions are acidic because they both contain hydrogen.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Needthe meter is the standard unit for: 1) height 2) length 3) weight 4) mass
Answers: 3

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2


Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2
You know the right answer?
The image above shows what happens when hydrochloric acid (HCl) and sodium hydroxide (NaOH) are mixe...
Questions

Spanish, 30.05.2021 21:20

Geography, 30.05.2021 21:20

Social Studies, 30.05.2021 21:20

Mathematics, 30.05.2021 21:20


Mathematics, 30.05.2021 21:20

Mathematics, 30.05.2021 21:20




Physics, 30.05.2021 21:20

Business, 30.05.2021 21:20

Mathematics, 30.05.2021 21:20

History, 30.05.2021 21:20


Mathematics, 30.05.2021 21:20




Chemistry, 30.05.2021 21:20



