

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1


Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1

Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2
You know the right answer?
4. Balance the following equation. Then determine the number of mols of Nitrogen formed given the va...
Questions


Mathematics, 09.10.2019 02:30












English, 09.10.2019 02:30

Social Studies, 09.10.2019 02:30




Mathematics, 09.10.2019 02:30

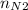 = 4 × 2 ÷ 4 = 2
= 4 × 2 ÷ 4 = 2 

