Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Pls! plant cells and animal cells were observed under a microscope. the characteristics of two cells are listed below. cell p: does not capture sunlight cell q: has cytoplasm but no chloroplast which statement about the two cells is correct? cell q also has a cell wall. cell q also has large vacuole. cell p also has a large vacuole. cell p also has a cell membrane.
Answers: 1

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2

Chemistry, 23.06.2019 04:00
Which of these are physical changes in matter? check all that apply boiling water a pencil being sharpened exploding dynamite freezing water rotting cheese
Answers: 1

Chemistry, 23.06.2019 16:30
If 950 joules of heat are needed to increase the temperature of 45.6 grams of an unknown metal from 25.0 celsius to 90.0 celsius, what is the specific heat capacity of the metal in j/g celsius
Answers: 2
You know the right answer?
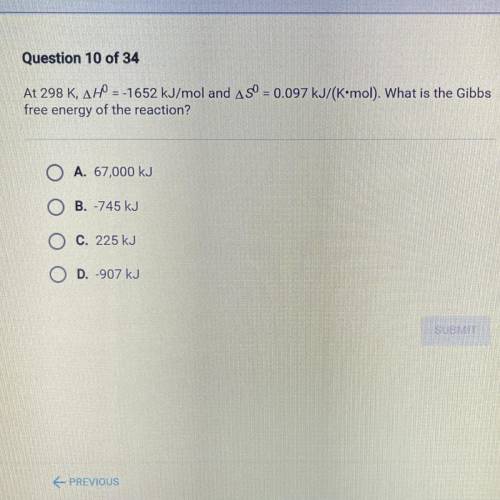
At 298 K, AH = -1652 kJ/mol and ASO = 0.097 kJ/(K•mol). What is the Gibbs
free energy of the react...
Questions

Mathematics, 08.02.2021 03:50

Chemistry, 08.02.2021 04:00

Advanced Placement (AP), 08.02.2021 04:00

Mathematics, 08.02.2021 04:00


Mathematics, 08.02.2021 04:00

Business, 08.02.2021 04:00

Mathematics, 08.02.2021 04:00

Mathematics, 08.02.2021 04:00

Chemistry, 08.02.2021 04:00

Mathematics, 08.02.2021 04:00


Mathematics, 08.02.2021 04:00

Mathematics, 08.02.2021 04:00

Mathematics, 08.02.2021 04:00

Mathematics, 08.02.2021 04:00

Mathematics, 08.02.2021 04:00

Mathematics, 08.02.2021 04:00