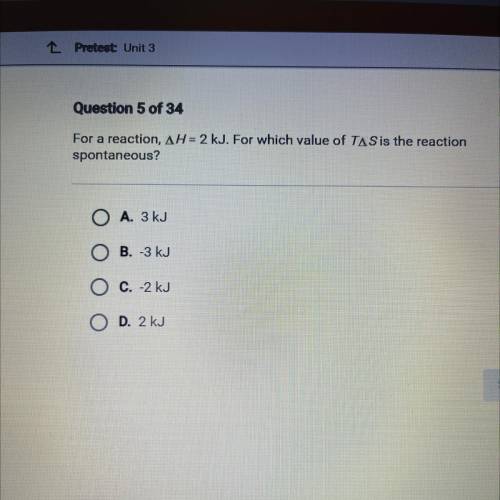
For a reaction, AH = 2 kJ. For which value of TAS is the reaction
spontaneous?
...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 23.06.2019 00:20
What type of context clue you understand the meaning of quandary?
Answers: 3

Chemistry, 23.06.2019 06:30
Which of the following is true about the products formed during photosynthesis? (5 points) select one: a. they have the same mass as the mass of reactants. b. they are the same set of compounds as the reactants. c. they have more mass than the mass of reactants. d. they are chemically the same as the reactants.
Answers: 1

Chemistry, 23.06.2019 07:30
How do you interpret a chromagram for what mixtures contain?
Answers: 1
You know the right answer?
Questions



History, 04.12.2020 20:30

Mathematics, 04.12.2020 20:30

Mathematics, 04.12.2020 20:30

Arts, 04.12.2020 20:30

English, 04.12.2020 20:30





Physics, 04.12.2020 20:30

Mathematics, 04.12.2020 20:30

Computers and Technology, 04.12.2020 20:30



Computers and Technology, 04.12.2020 20:30

Chemistry, 04.12.2020 20:30


Mathematics, 04.12.2020 20:30