
Chemistry, 04.05.2021 23:00 faithlopez209
PLEASE HELP, WILL GIVE BRAINLIEST
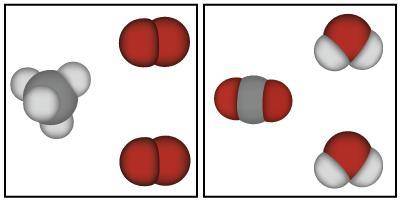
The image below shows models that represent the reactants and products of a chemical reaction.
Based on the image below, which of the following is true?
A. Mass could have either been gained or lost during this chemical reaction because atoms always change mass when they react.
B. Mass was lost in this chemical reaction because the atoms in the reactants are smaller than the atoms in the products.
C. Mass was conserved in this chemical reaction because the same atoms are present in both the products and the reactants.
D. Mass was gained in this chemical reaction because there are more atoms in the products than there were in the reactants.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 10:00
Which sentence about particles in matter is true? a. atoms are present in solids and liquids but not in gases. b. the particles of matter are in constant motion. c. the same kinds of atoms are found in different elements. d. when a solid changes to a liquid, the sizes of the particles change.
Answers: 1

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 21:30
If you burn 46.6 g of hydrogen and produce 416 g of water, how much oxygen reacted
Answers: 3
You know the right answer?
PLEASE HELP, WILL GIVE BRAINLIEST
The image below shows models that represent the reactants and pr...
Questions



Mathematics, 17.02.2020 00:42

History, 17.02.2020 00:42

Mathematics, 17.02.2020 00:42

History, 17.02.2020 00:42


Physics, 17.02.2020 00:43

Mathematics, 17.02.2020 00:43


Biology, 17.02.2020 00:44







History, 17.02.2020 00:45




