
Chemistry, 04.05.2021 18:10 myalee1419
No links I will report
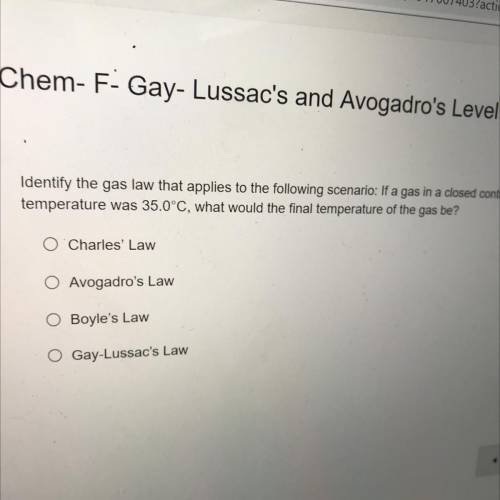
Identify the gas law that applies to the following scenario: If a gas in a closed container is pressurized from 18.0 atm to 14.0 atm and its original
temperature was 35,0°C, what would the final temperature of the gas be?
Charles Law
Avogadro's Law
Boyle's Law
Gay-Lussac's Law

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 06:00
When a spring is compressed, the energy changes from kinetic to potential. which best describes what is causing this change?
Answers: 3

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1
You know the right answer?
No links I will report
Identify the gas law that applies to the following scenario: If a gas in a...
Questions

Mathematics, 01.01.2020 11:31

Mathematics, 01.01.2020 11:31

Mathematics, 01.01.2020 11:31




Mathematics, 01.01.2020 11:31




Mathematics, 01.01.2020 11:31



Mathematics, 01.01.2020 11:31


English, 01.01.2020 11:31



Mathematics, 01.01.2020 11:31

Mathematics, 01.01.2020 11:31



