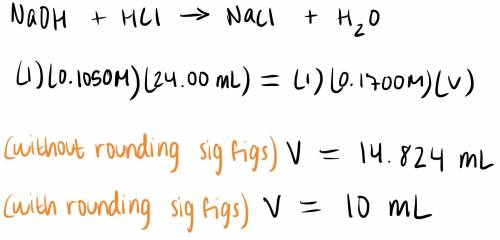Chemistry, 04.05.2021 05:00 marieknight689
What volume of 0.1700 M NaOH is required to titrate 24.00 mL of 0.1050 M HCI?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1


Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1
You know the right answer?
What volume of 0.1700 M NaOH is required to titrate 24.00 mL of 0.1050 M HCI?...
Questions

Social Studies, 14.12.2019 17:31

Biology, 14.12.2019 17:31


Social Studies, 14.12.2019 17:31

Biology, 14.12.2019 17:31


Mathematics, 14.12.2019 17:31



Social Studies, 14.12.2019 17:31

Mathematics, 14.12.2019 17:31




History, 14.12.2019 17:31