
Chemistry, 03.05.2021 21:10 estefaniapenalo
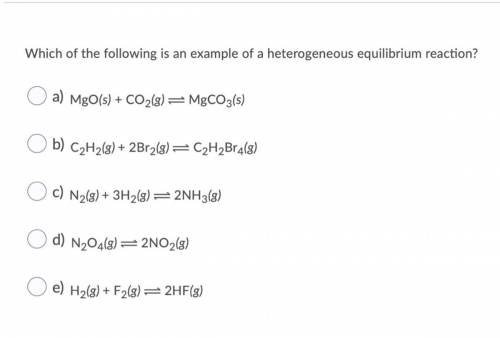
Which of the following is an example of a heterogeneous equilibrium reaction? O a) MgO(s) + CO2(g) = MgCO3(s) O b) C2H2(g) + 2Br2(g) = C2H2Br4(8) O c) N2(g) + 3H2(g) = 2NH3(8) O d) N204(8) =2NO2(8) O e) H2(g) + F2(8)=2HF(3)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2
You know the right answer?
Which of the following is an example of a heterogeneous equilibrium reaction? O a) MgO(s) + CO2(g) =...
Questions



History, 15.04.2020 23:15


Mathematics, 15.04.2020 23:15


English, 15.04.2020 23:15



Computers and Technology, 15.04.2020 23:15

Mathematics, 15.04.2020 23:15



Mathematics, 15.04.2020 23:15

History, 15.04.2020 23:15

Mathematics, 15.04.2020 23:15

Mathematics, 15.04.2020 23:15






