
Chemistry, 03.05.2021 20:30 HaJEReMY5170
The graph shown shows the rates of a reaction between zinc and hydrochloric acid to produce hydrogen gas. It was conducted under 3 different conditions. The rate of the reaction is shown by the slope of each line. If the reaction was conducted with solid zinc twice and crushed/powdered zinc once. Which line most likely shows the rate for the reaction with the crushed/powdered zinc?
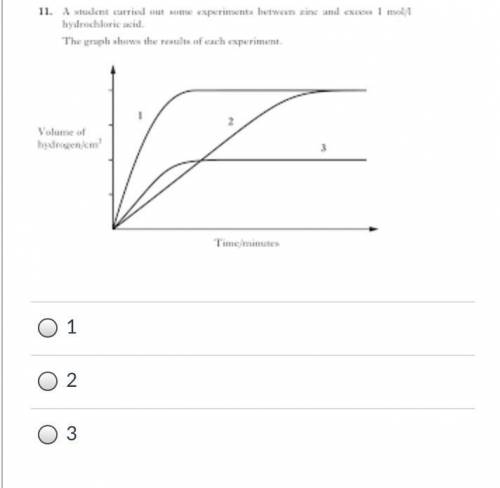

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
In which direction will the following reaction go if the standard reduction potentials are 0.80 v for ag/ag+ and –0.44 v for fe/fe2+? ag+ + fe → ag + fe2+ a.)forward b.)the reaction cannot occur. c.) not enough information is given. d.) reverse
Answers: 1

Chemistry, 21.06.2019 18:30
Covalent network solids typically have melting points and boiling points. the chemical formula of a network solid indicates in the molecule.
Answers: 3

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1
You know the right answer?
The graph shown shows the rates of a reaction between zinc and hydrochloric acid to produce hydrogen...
Questions

Mathematics, 18.10.2021 08:30

Mathematics, 18.10.2021 08:30

Mathematics, 18.10.2021 08:30


English, 18.10.2021 08:30




Biology, 18.10.2021 08:30


English, 18.10.2021 08:30


Mathematics, 18.10.2021 08:30

English, 18.10.2021 08:30

English, 18.10.2021 08:30


Mathematics, 18.10.2021 08:30


Mathematics, 18.10.2021 08:30

Mathematics, 18.10.2021 08:30



