
Chemistry, 03.05.2021 19:10 joshua13338
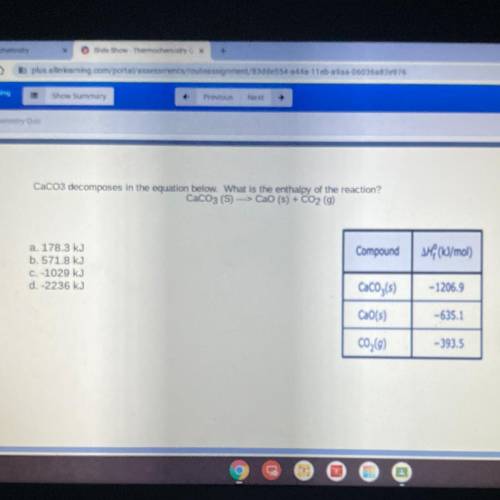
CaCo3 decomposes in the equation below. What is the enthalpy of the reaction?
CaCO3 (S) ---> CaO (s) + CO2 (g)
a. 178.3 kJ
b. 571.8 kJ
C. -1029 kJ
d. -2236 kJ

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:40
Achemistry student weighs out of phosphoric acid , a triprotic acid, into a volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with solution. calculate the volume of solution the student will need to add to reach the final equivalence point. round your answer to significant digits.
Answers: 3

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Chemistry, 23.06.2019 00:00
How many peaks will be present in a mass spectrum for brcl?
Answers: 1

Chemistry, 23.06.2019 00:00
What does an electron configuration for an atom relate to the atoms placement on the periodic table
Answers: 2
You know the right answer?
CaCo3 decomposes in the equation below. What is the enthalpy of the reaction?
CaCO3 (S) ---> Ca...
Questions


Social Studies, 16.10.2020 22:01


Mathematics, 16.10.2020 22:01



Mathematics, 16.10.2020 22:01


Computers and Technology, 16.10.2020 22:01

Chemistry, 16.10.2020 22:01

Health, 16.10.2020 22:01



Physics, 16.10.2020 22:01

Mathematics, 16.10.2020 22:01




Mathematics, 16.10.2020 22:01

Chemistry, 16.10.2020 22:01



