
Chemistry, 03.05.2021 19:00 johndous3698
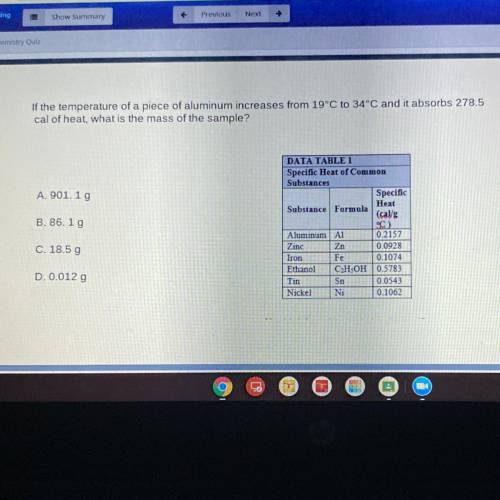
If the temperature of a piece of aluminum increases from 19°C to 34°C and it absorbs 278.5
cal of heat, what is the mass of the sample?
A. 901.19
B. 86.19
DATA TABLE 1
Specific Heat of Common
Substances
Specific
Heat
Substance Formula
(cal/g
°C
Aluminum A1 0.2157
Zinc Zn 0.0928
Iron Fe 0.1074
Ethanol C2H5OH 0.5783
Tin Sn 0.0543
Nickel Ni 0.1062
C. 18.5 g
D. 0.012 g

Answers: 1


Another question on Chemistry




Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2
You know the right answer?
If the temperature of a piece of aluminum increases from 19°C to 34°C and it absorbs 278.5
cal of...
Questions

Computers and Technology, 14.05.2021 22:30



Physics, 14.05.2021 22:30

Mathematics, 14.05.2021 22:30




Spanish, 14.05.2021 22:30

Advanced Placement (AP), 14.05.2021 22:30

Social Studies, 14.05.2021 22:30


Mathematics, 14.05.2021 22:30

Mathematics, 14.05.2021 22:30

Mathematics, 14.05.2021 22:30

English, 14.05.2021 22:30

Mathematics, 14.05.2021 22:30

Mathematics, 14.05.2021 22:30




