
Chemistry, 03.05.2021 18:50 datgamer13
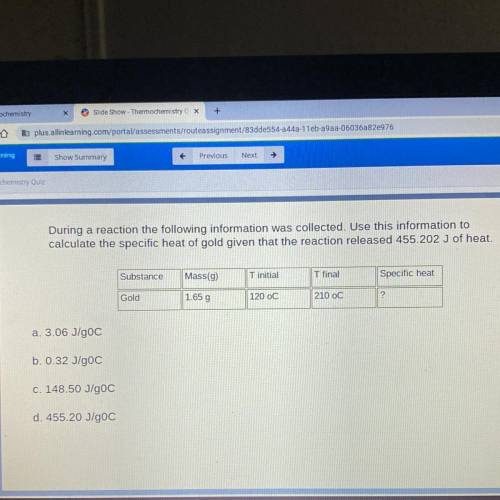
During a reaction the following information was collected. Use this information to
calculate the specific heat of gold given that the reaction released 455.202 J of heat.
Substance
Mass(9)
T initial
T final
Specific heat
Gold
1.65 g
120 oC
210 OC
2
a. 3.06 J/gOC
b. 0.32 J/goC
c. 148.50 J/goC
d. 455.20 J/goC

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2

Chemistry, 23.06.2019 01:30
What happens to the concentration of hydronium ions as the ph of a solution increases? a. hydronium ion concentration stays the same b. hydronium ion concentration decreases c. hydronium ion concentration increases
Answers: 1
You know the right answer?
During a reaction the following information was collected. Use this information to
calculate the s...
Questions

Mathematics, 25.06.2019 13:30








Mathematics, 25.06.2019 13:30

Mathematics, 25.06.2019 13:30

Mathematics, 25.06.2019 13:30


Mathematics, 25.06.2019 13:30

Mathematics, 25.06.2019 13:30

Mathematics, 25.06.2019 13:30

Mathematics, 25.06.2019 13:30



Mathematics, 25.06.2019 13:30



