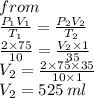Chemistry, 03.05.2021 18:30 katherine3084
3. At 10.0°C and 2.00 atm, the volume of the gas is 75.0 mL. If the temperature of the gas increases to
35.0°C and the pressure decreases to 1.00 atm, what is the new volume?
a. 1630 mL
b. 138 ml
c. 163 mL
d. 0.0017mL

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3
You know the right answer?
3. At 10.0°C and 2.00 atm, the volume of the gas is 75.0 mL. If the temperature of the gas increases...
Questions

History, 27.02.2020 03:33






Mathematics, 27.02.2020 03:34

History, 27.02.2020 03:34










Mathematics, 27.02.2020 03:34