Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:00
1) how many electrons are in each energy level of the following elements? a. he b. na c. na d. ne 2) how many valence electrons are percent in the following atoms? a. s b. mg c. be d. cl 3) which of the following elements are stable as atoms? a. he b. o c. cl d. ar if you are able to provide the work as to how you got the answers that would be greatly appreciated. : )
Answers: 1


Chemistry, 21.06.2019 23:00
Esign techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 3

Chemistry, 22.06.2019 21:30
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1
You know the right answer?
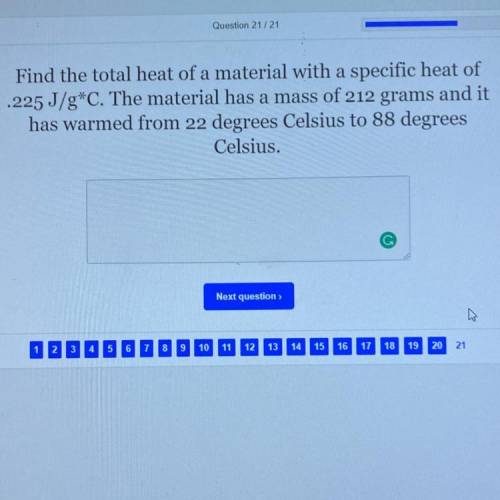
Find the total heat of a material with a specific heat of
.225 J/g*C. The material has a mass of 2...
Questions

History, 15.10.2019 12:00

Mathematics, 15.10.2019 12:00

Mathematics, 15.10.2019 12:00



English, 15.10.2019 12:00

Social Studies, 15.10.2019 12:00

History, 15.10.2019 12:00


Mathematics, 15.10.2019 12:00


Mathematics, 15.10.2019 12:00







Mathematics, 15.10.2019 12:00

English, 15.10.2019 12:00