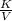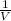What will happen if the pressure of the system is raised to 1.5 atmospheres and the temperature remains same?
The piston will move upwards because the number of moles of the gas particles will increase.
The piston will move downwards because the gas particles will occupy less volume than before.
The piston will move upwards because the gas particles will hit the walls of the container with increased force.
The piston will move downwards because the gas particles will start moving randomly in all directions, pulling the piston down.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Is this a scientific model? use complete sentences to explain why or why not. a graphic organizer showing the water cycle
Answers: 3


Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1
You know the right answer?
What will happen if the pressure of the system is raised to 1.5 atmospheres and the temperature rema...
Questions


Mathematics, 27.04.2021 22:10

Mathematics, 27.04.2021 22:10


Social Studies, 27.04.2021 22:10

Mathematics, 27.04.2021 22:10


Mathematics, 27.04.2021 22:10




Mathematics, 27.04.2021 22:10


Mathematics, 27.04.2021 22:10

English, 27.04.2021 22:10


Mathematics, 27.04.2021 22:10


History, 27.04.2021 22:10

Mathematics, 27.04.2021 22:10