
Chemistry, 02.05.2021 18:20 yenielrivera30
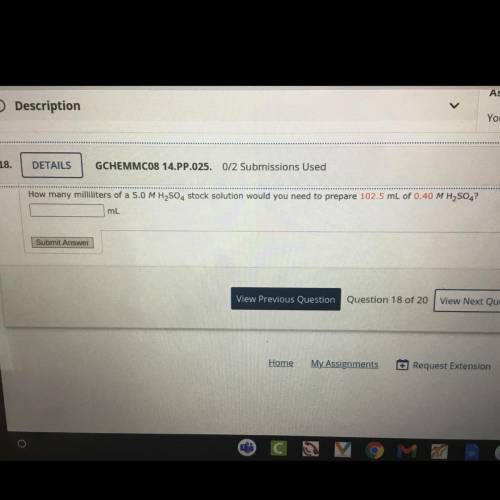
How many milliliters of a 5.0 MH2SO4 stock solution would you need to prepare 102.5 mL of 0.40 MH2SO4?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 23.06.2019 10:30
Identify the limiting reactant when 9.65-g h2so4 reacts with 6.10-g of naoh.the equation is h2s04 + 2naoh = 2h2o + na2so4• what is the theoretical yield of na2so4, in grams? • how much of the excess reagent will remain after the reaction has been completed? • if 10.5-g of na2so4 are actually recovered experimentally, what is the percent yield?
Answers: 3

Chemistry, 23.06.2019 12:30
Question 1 (true/false worth 4 points) (03.06 lc) an induced dipole occurs when a molecule's moving electrons are briefly more concentrated in one place than another, causing the molecule to become temporarily polarized. true false
Answers: 2

You know the right answer?
How many milliliters of a 5.0 MH2SO4 stock solution would you need to prepare 102.5 mL of 0.40 MH2SO...
Questions


Mathematics, 09.10.2019 03:00



Chemistry, 09.10.2019 03:00

Mathematics, 09.10.2019 03:00

Mathematics, 09.10.2019 03:00





Mathematics, 09.10.2019 03:00

Social Studies, 09.10.2019 03:00

Social Studies, 09.10.2019 03:00

English, 09.10.2019 03:00


Mathematics, 09.10.2019 03:00

Biology, 09.10.2019 03:00


Biology, 09.10.2019 03:00



