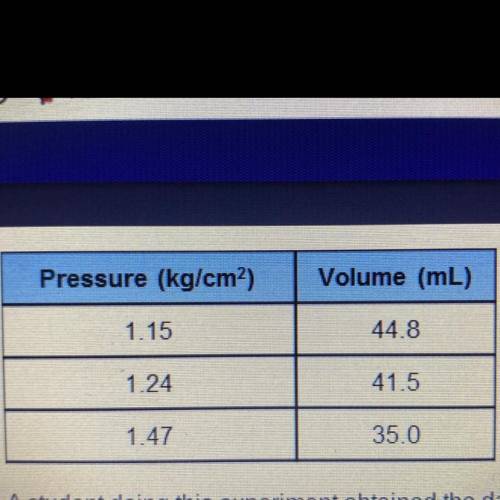
Suppose that the student took one more volume
reading of 24.2 mL but forgot to measure the
p...

Chemistry, 01.05.2021 08:50 carlosiscr7
Suppose that the student took one more volume
reading of 24.2 mL but forgot to measure the
pressure.
Compute the pressure expected for that
volume. kg/cm2?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3
You know the right answer?
Questions



Mathematics, 28.11.2021 01:00

Computers and Technology, 28.11.2021 01:00


Mathematics, 28.11.2021 01:00





Chemistry, 28.11.2021 01:00



Mathematics, 28.11.2021 01:00


Biology, 28.11.2021 01:00


Mathematics, 28.11.2021 01:00

Mathematics, 28.11.2021 01:00



