
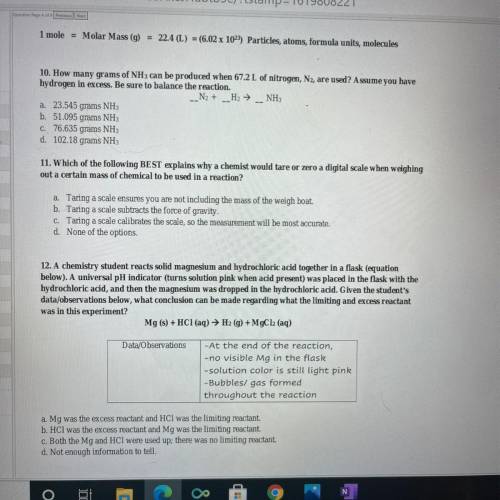
10. How many grams of NH 3 can be produced when 67.2 L of nitrogen, , are used? Assume you have N 2 hydrogen in excess. Be sure to balance the reaction. 12. A chemistry student reacts solid magnesium and hydrochloric acid together in a flask (equation below). A universal pH indicator (turns solution pink when acid present) was placed in the flask with the hydrochloric acid, and then the magnesium was dropped in the hydrochloric acid. Given the student's data/ observations below, what conclusion can be made regarding what the limiting and excess reactant was in this experiment?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Chemistry, 23.06.2019 07:30
Type the letter that represents the correct location for each particle type below. the neutron is found at __ the electron is found at __ the proton is found at __
Answers: 2

Chemistry, 23.06.2019 09:30
If the solubility of a gas in water is 1.22g/2.75 atm, what is it’s solubility (in g/l) at 1.0 atm
Answers: 1
You know the right answer?
10. How many grams of NH 3 can be produced when 67.2 L of nitrogen, , are used? Assume you have N 2...
Questions




History, 09.10.2019 07:50

Social Studies, 09.10.2019 07:50



Spanish, 09.10.2019 07:50


English, 09.10.2019 07:50





Computers and Technology, 09.10.2019 07:50


Chemistry, 09.10.2019 07:50





