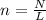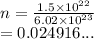1 point
How many moles of magnesium is 1.50 x 10^22 atoms of magnesium?...

Chemistry, 30.04.2021 20:40 baileymtamayo
1 point
How many moles of magnesium is 1.50 x 10^22 atoms of magnesium?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

Chemistry, 22.06.2019 19:20
15. which of the following is not human-caused groundwater pollution? a. water in an aquifer dissolves elements such as arsenic and mercury from surrounding rock. b. water in an aquifer is contaminated by leachate that seeps into the ground from a landfill. c. water in an aquifer becomes polluted with chemicals used in hydraulic fracturing, or fracking. d. water in an aquifer absorbs harmful bacteria from the drainage field of a septic tank.
Answers: 1

Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1

Chemistry, 23.06.2019 00:50
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3
You know the right answer?
Questions



Biology, 29.11.2021 01:20


Chemistry, 29.11.2021 01:20


Advanced Placement (AP), 29.11.2021 01:20

English, 29.11.2021 01:20

History, 29.11.2021 01:20


Computers and Technology, 29.11.2021 01:20

Mathematics, 29.11.2021 01:20


History, 29.11.2021 01:20

Engineering, 29.11.2021 01:20




Mathematics, 29.11.2021 01:20