
Chemistry, 30.04.2021 19:40 james22000
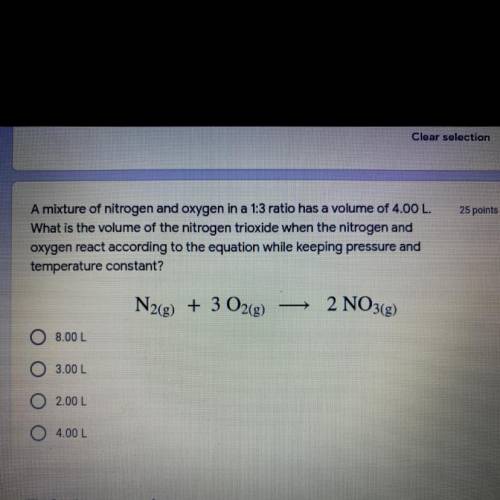
A mixture of nitrogen and oxygen in a 1:3 ratio has a volume of 4.00 L.
What is the volume of the nitrogen trioxide when the nitrogen and
oxygen react according to the equation while keeping pressure and temperature constant?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 10:00
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1


Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3
You know the right answer?
A mixture of nitrogen and oxygen in a 1:3 ratio has a volume of 4.00 L.
What is the volume of the...
Questions


Biology, 22.02.2021 23:30

Mathematics, 22.02.2021 23:30

Mathematics, 22.02.2021 23:30





Spanish, 22.02.2021 23:30

Mathematics, 22.02.2021 23:30

Mathematics, 22.02.2021 23:30








Mathematics, 22.02.2021 23:30



