
Chemistry, 30.04.2021 15:40 charlesmb7985
If the volume occupied by the gas molecules shown below were doubled, what would happen to the pressure they exert? (Assume constant temperature.)
A. 0.25 atm
B. 0.50 atm
C. 1.00 atm
D. 2.00 atm
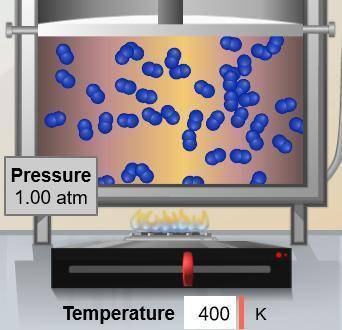

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 19:40
What type of electromagnetic waves does the human eye see as the colors red blue or green a visible light waves b radio waves c infrared waves d microwaves
Answers: 1

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
You know the right answer?
If the volume occupied by the gas molecules shown below were doubled, what would happen to the press...
Questions








Mathematics, 05.12.2019 20:31

English, 05.12.2019 20:31


Mathematics, 05.12.2019 20:31









Mathematics, 05.12.2019 20:31



