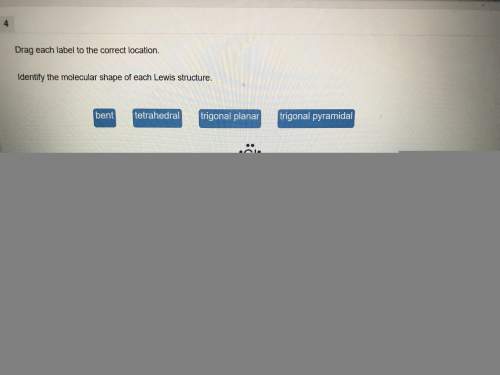
Chemistry, 29.04.2021 22:30 lanaiheart7
A gas has a pressure of 900.0 millibars at a temperature of 30.0°C. If the volume is unchanged but the temperature is increased to 80.0°C, what is the new pressure of the gas?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1

Chemistry, 23.06.2019 05:20
Explain how global warming could have affected yellowstone frog and salamander habitat's, resulting in changes in the populations of these species
Answers: 2

Chemistry, 23.06.2019 09:30
How many moles of na2s2o3 are needed to react with 0.12mol of cl2? show work.
Answers: 1
You know the right answer?
A gas has a pressure of 900.0 millibars at a temperature of 30.0°C. If the volume is unchanged but t...
Questions




Mathematics, 06.07.2019 12:00




Health, 06.07.2019 12:00





Computers and Technology, 06.07.2019 12:00


Mathematics, 06.07.2019 12:00

Physics, 06.07.2019 12:00

Mathematics, 06.07.2019 12:00


Geography, 06.07.2019 12:00